eIRB Basics
Taylor Bates, MD, Capt, USAF, MC
Daniel J. Cognetti, MD, CPT, USA, MC
Gaining Access
Gaining access to eIRB consists of several steps and typically takes 5-10 business days. After approval an email will be sent to the email address associated with your account request. There are two options for requesting new user accounts.
- Using your CAC to register by going to https://eirb.csd.disa.mil
- Click the button under “Authenticate your DoD CAC, VA PIV, or ECA certificate” (Figure 1)
- Register your CAC by completing the required fields
- Verify your official .mil email address is entered
- Verify the site where you plan to conduct research
- Select “request account”
- Your account request will be approved or denied by your local site administrator
- eIRB will contact the requestor for Cyber Security training certificate
- A Biomedical CITI training certificate may also be requested (www.citiprogram.org)
- Initiate a new user account through the DHA Global Service Center via email ([email protected]) or phone 800-600-9332
- Be prepared to provide information located on your CAC
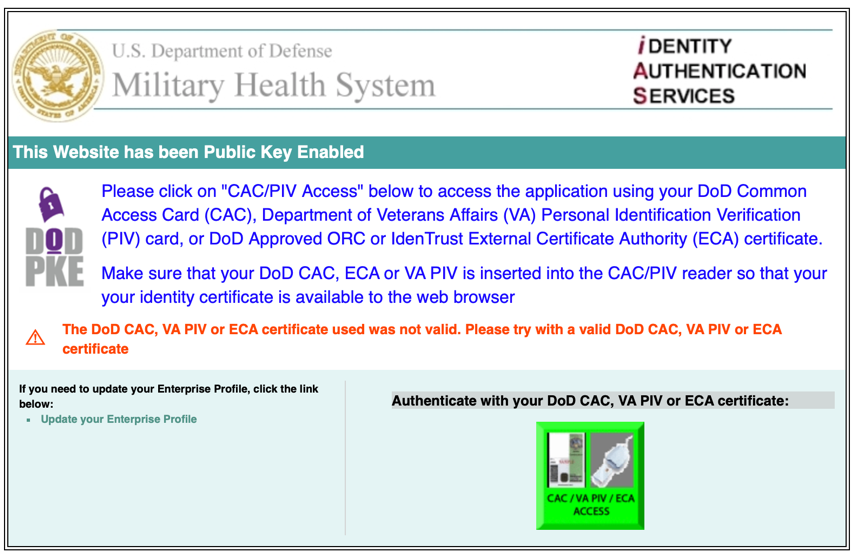
Figure 1 https://eirb.csd.disa.mil
Starting a Protocol
- Log into eIRB with your CAC on the eIRB website (Figure 1): https://eirb.csd.disa.mil
- Select “Add a New Protocol” on the home screen (Figure 2)
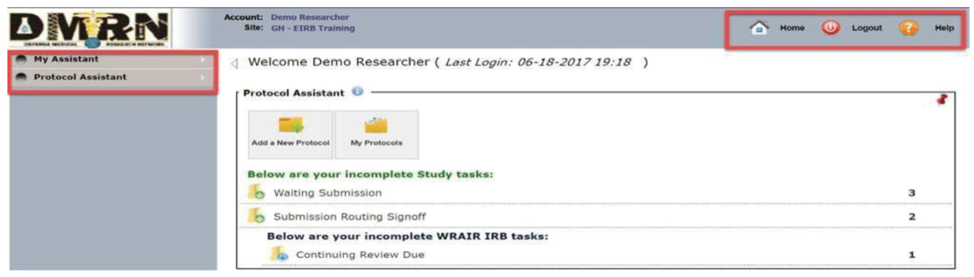 Figure 2 eIRB Home Screen Figure 2 eIRB Home Screen
3. Follow the prompts to begin a new protocol
4. Ensure you have uploaded all necessary supplemental documents (“s-forms”; i.e., Conflict of Interest, Key Study Personnel, etc.
- Available on the eIRB website and through your institution
- Required documents vary depending on the type of study and study requirements, check with your local IRB office when clarification is needed
- A Curriculum Vitae from each investigator is required
- A Biomedical CITI training certificate from each investigator is required when interacting with subjects or patient data (www.citiprogram.org)
- Documents are uploaded at the end of the protocol submission process
- See A Researcher’s Guide to Navigating the Approval Process for more information
Protocol Processing
- The IRB typically reviews protocols once a month. Knowing your institution’s submission deadline for each period can help avoid delays.
- Your protocol may be returned after an initial review for corrections (i.e., formatting) prior to an official IRB review
- Corrections and/or clarification may be requested after an official IRB review is conducted
- An email notification will be sent to inform you that the document has been returned
- Log into the eIRB website and open your protocol submission to view requested submission corrections and/or clarification
- See the eIRB Submission Corrections Guide for further details
- Routinely check the eIRB website for updates regarding your submissions. Investigators are not always notified of protocol acceptance.
Tips and Tricks
- Use a Word Doc template to write your protocol then copy and paste each section into the eIRB
- Allows easier exchanging and editing when multiple investigators are involved
- No CAC access required
- Allows use of a citation manager (i.e., EndNote, Zotero)
- Downloadable templates are available on the eIRB website
- Email or call the IRB office with questions or to schedule a meeting with a member of the IRB if you have trouble writing a protocol, addressing stipulations or have not received correspondence on a protocol for an extended period of time
- Statisticians are usually available through the GME office at training institutions and are helpful when writing the statistical analysis section
- Images and illustrations can be referenced within the protocol and uploaded during the submission process
- These can help the IRB members (mostly non-orthopaedic specialties) better understand your study
- eIRB forms and templates are available on the eIRB website (https://eirb.csd.disa.mil)
|






